Do I need a water softener system?
Soft water cuts energy costs, uses less soap, makes housework easier, and helps you look and feel better. Here’s how a softener works and how to tell if your home needs one.
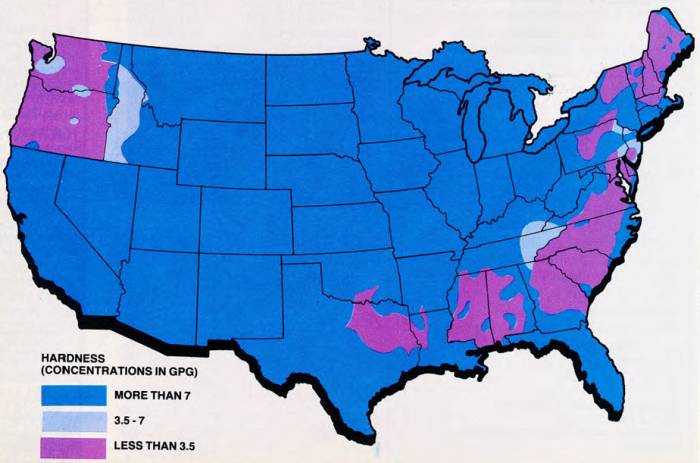
Hard water is a problem that affects a majority of the people in the United States. Water in 85 percent of the country is hard enough that softening is either required or recommended. Take a look at the map of hard water concentration to see how your area is rated.
Before you read more on how a water softener works and without taking a chemistry course, let’s see how water hardness occurs and the problems associated with it. This will help in determining if you need (or want) a water softener.
What is hard water?
Hard water is how we refer to water that contains the hardness minerals calcium (Ca) and magnesium (Mg). Rain water, by contrast, is naturally soft because these minerals aren’t present. That’s why people used to collect it and use it for washing clothes.
Rain water doesn’t stay soft for long. As it filters down through the earth and becomes part of the ground water supply, it picks up minerals of hardness (Ca and Mg).
Why be concerned?
The two main problems associated with hard water are the formation of soap curd and scale. These conditions can damage appliances such as dishwashers, make clothing dull and dingy looking, and clog water pipes.
Soap curd is an insoluble material formed when the hardness minerals react with soaps and detergents. Soap curds deposit themselves on fabrics, dulling the colors and making whites look grey or yellow. They also cling to the fabric fibers, making them brittle and shortening the life of the material.
Because soaps and detergents combine with the hardness minerals and form soap curds, you end up using more soap and detergent. Hard water also leaves behind soap scum rings and rusty stains. This makes cleaning more difficult and time-consuming.
Scale formation is the other serious problem. Scale is formed when hard water is heated. Scale build-up gradually coats the interior of pipes, water heaters, boilers and appliances, reducing water flow and efficiency.

Two other concerns:
- just as hard water leaves a film behind on clothes, there’s a similar film residue on your skin after bathing, and
- hard water sometimes tastes bad.
Why hard water is costly
A study by the University of New Mexico showed that it took much more energy to heat hard water. Gas water heaters warming soft water used 29 percent less energy than the same heaters warming hard water. Electric water heaters using salt water consumed 21 percent less energy.
You might be able to shrug your shoulders at that and absorb the one-third greater cost, but there’s another expense: extra time spent on housework. The staining of sinks, toilets and tubs makes them considerably harder to clean; and if the stain can’t be removed, your only alternative is replacing the fixture.
How to combat hard water
There are several ways to reduce water hardness: deionization, distillation, reverse osmosis and cation exchange. The method that’s used most often, and best suited for residential use is cation exchange.
Understanding cation exchange
The principles of a cation exchange system are not very complex. A cation is a positively charged ion. An ion is an electrically charged atom or group of atoms.
To soften water, the hardness ions (Ca and Mg) are exchanged with sodium (Na) ions. This exchange occurs in the resin bed, during the softening cycle.
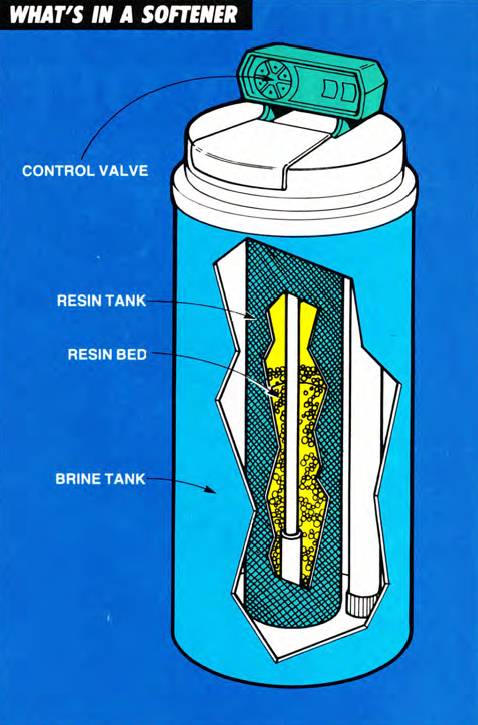
What’s in a softener?
An ion exchange water softener is made up of four basic components:
- resin tank: It contains the resin bed.
- resin bed: This is where the real action takes place. The resin bed is made up of a tiny bead-like material composed of styrene and divinylbenzene. (The actual resin beads are less than 1/32 of an inch in diameter.) The resin beads attract and hold positively charged ions (Na), but will give them up (exchange them) whenever the bead encounters another positively charged ion (Ca and Mg), for which it has greater affinity. Think of it as “The Dating Game.”
- brine tank: This holds the water and dissolved salt solution (brine) that is necessary to perform the ion exchange.
- control valve: It determines the direction of the flow of water through the resin tank or from one location to another during the various softening cycles.
There comes a point when the resin can no longer soften the water. It becomes exhausted. If no new chemical reaction (recharging) is begun, the Ca and Mg ions flow through untouched and the water is no longer softened.
The recharging process
In recharging (regenerating) the water softener, what you’re doing is reversing the ion exchange mention. The Ca and Mg ions are driven off of the resin beads and replaced with Na ions.
Accomplishing this regeneration requires that the resin beads be washed with a strong salt water solution (referred to as “brine”). The excessive concentration of salt forces the Ca and Mg ions to be released and they’re discharged as waste during the backwashing cycle.
The beads are then ready to once again remove Ca and Mg from the water.
Sounds like a lot of work?
In the not too distant past, recharging the softener was a chore. Today, however, the softeners are fully automatic and extremely reliable. The regeneration process comes on automatically, with your only task the occasional filling of the salt storage tank.
Do you need a water softener?
First, you should have your water supply’s hardness level tested. If it’s tested by someone who sells softeners, you can bet you’ll need to buy one. If it’s tested by an independent testing laboratory, or by a firm recommended by your county or municipal health department, maybe not. (You can perform a preliminary hardness test, see T-I-Y section below).
Most manufacturers of water softening equipment will test your water free of charge. (Remember, though, they’re in the business to sell, so be ready to endure the sales pitch.) The salesperson will come to your house, draw a sample of tap water and usually be able to determine the water hardness right in your home.
Another source is through your local health department. They should be able to recommend a testing lab that will provide you with accurate test results for a nominal fee. (A water hardness test usually runs about $20-30). One nice thing about these folks is they usually aren’t trying to sell you a water softener.
What the test tells you
Water hardness is expressed in “grains per gallon” (abbreviated as GPG). The term is a measurement of the number of grains of a given substance in one US gallon of water. Water supplies in this country typically run from 3 to 50 GPG.
- 0 to 1 GPG: soft water
- 1 to 3.5 GPG: slightly hard water
- 3.5 to 7 GPG: moderately hard water
- 7+ GPG: hard or very hard water
The water analysis you have performed will tell you the GPG figure and thus whether a softener is called for.
What size softener
After you’ve learned your water’s GPG, you need to determine your daily household water use before you can choose the right softener. To do this, multiply 75 (average daily per person gallons of water used) by the number of people in your household.
For example, a 4-person household would average 300 gals. per day. Multiplying the 300 gallons x GPG hardness of your Water (20 GPG is common in many areas), gives you a figure of 6,000 GPG total hardness per day.
Water softener capacities are given in terms of the number of grains of hardness they will remove between successive regenerations. The typical household softener’s capacity is between 20,000-30,000 grains. It’s recommended that a softener have enough capacity to last at least three days between regenerations.
Using the example of 6,000 GPG, a 3-day capacity means 18,000 grains. Thus, a softener with a 20,000 resin bed capacity would meet your needs.
Added sodium concerns
The harder the water supply being softened, the more sodium that’s added to the water. If you’re concerned about the added sodium in your body, or if you’re already on a low sodium diet, check with your doctor before installing a water softener.
You can prevent adding sodium to your water intake by having soft water in the hot water line only. Then you’re able to drink and cook with unsoftened, cold water.
T-I-Y: Test It Yourself
There’s a test for water hardness that you can do without fancy equipment. It’s not the most sophisticated test, and you won’t get specific numbers, but you’ll get an idea about the hardness of your water supply.
The test requires three items: a glass jar, with lid; some pure detergent or bar soap (Ivory soap or detergent works well); and unsoftened, cold tap water.
Fill the jar about half-full with cold, unsoftened tap water. Next, sprinkle just a few flakes of detergent, or scrape a few shavings of bar soap into the water. Place the lid securely on the jar and shake the water and detergent/soap until a lather or foam appears on top of the water.
If there’s little or no lather, the water would be considered hard. Now, dip your finger into the water. Hard water will leave soap deposits, or soap curd, on your finger and the inside of the jar. If this is your situation, you’ll probably want your water hardness tested more scientifically.
Magnetize your water for softness? Don’t bet on it
Water conditioning gadgets and gimmicks appear throughout the country on a regular basis. They bring to mind the old “Cure All Elixers.” Use them, and your problems are solved.
Baloney!
The water magnet is one of these oft-promoted wonder cures. It’s attached to the hot or cold water line and claims to magnetically charge the water and lock the hardness minerals in each molecule. For best results, of course, the manufacturer recommends you buy a magnet for both hot and cold lines. Besides, they sell for $400 each!
One advertising illustration actually shows confused water molecules running helter skelter in the water line. But, after they pass through the section of pipe with the magnetizer, they come out in neat little rows, like toy soldiers.
The independent tests on these products show one thing: They don’t work.
There have been numerous tests conducted to check the validity of these claims. The South Dakota School of Mines and Technology found no change in the physical and/or chemical properties of water treated with permanent magnetic devices.
Just remember, if it sounds too good to be true, it probably is.
Soft water cuts energy costs, uses less soap, makes housework easier, and helps you look and feel better. Here’s how a softener works and how to tell if your home needs one.
Hard water is a problem that affects a majority of the people in the United States. Water in 85 percent of the country is hard enough that softening is either required or recommended. Take a look at the map of hard water concentration to see how your area is rated.

Before you read more on how a water softener works and without taking a chemistry course, let’s see how water hardness occurs and the problems associated with it. This will help in determining if you need (or want) a water softener.
What is hard water?
Hard water is how we refer to water that contains the hardness minerals calcium (Ca) and magnesium (Mg). Rain water, by contrast, is naturally soft because these minerals aren’t present. That’s why people used to collect it and use it for washing clothes.
Rain water doesn’t stay soft for long. As it filters down through the earth and becomes part of the ground water supply, it picks up minerals of hardness (Ca and Mg).
Why be concerned?
The two main problems associated with hard water are the formation of soap curd and scale. These conditions can damage appliances such as dishwashers, make clothing dull and dingy looking, and clog water pipes.
Soap curd is an insoluble material formed when the hardness minerals react with soaps and detergents. Soap curds deposit themselves on fabrics, dulling the colors and making whites look grey or yellow. They also cling to the fabric fibers, making them brittle and shortening the life of the material.
Because soaps and detergents combine with the hardness minerals and form soap curds, you end up using more soap and detergent. Hard water also leaves behind soap scum rings and rusty stains. This makes cleaning more difficult and time-consuming.
Scale formation is the other serious problem. Scale is formed when hard water is heated. Scale build-up gradually coats the interior of pipes, water heaters, boilers and appliances, reducing water flow and efficiency.

Two other concerns:
- just as hard water leaves a film behind on clothes, there’s a similar film residue on your skin after bathing, and
- hard water sometimes tastes bad.
Why hard water is costly
A study by the University of New Mexico showed that it took much more energy to heat hard water. Gas water heaters warming soft water used 29 percent less energy than the same heaters warming hard water. Electric water heaters using salt water consumed 21 percent less energy.
You might be able to shrug your shoulders at that and absorb the one-third greater cost, but there’s another expense: extra time spent on housework. The staining of sinks, toilets and tubs makes them considerably harder to clean; and if the stain can’t be removed, your only alternative is replacing the fixture.
How to combat hard water
There are several ways to reduce water hardness: deionization, distillation, reverse osmosis and cation exchange. The method that’s used most often, and best suited for residential use is cation exchange.
Understanding cation exchange
The principles of a cation exchange system are not very complex. A cation is a positively charged ion. An ion is an electrically charged atom or group of atoms.
To soften water, the hardness ions (Ca and Mg) are exchanged with sodium (Na) ions. This exchange occurs in the resin bed, during the softening cycle.
What’s in a softener?
An ion exchange water softener is made up of four basic components:
- resin tank: It contains the resin bed.
- resin bed: This is where the real action takes place. The resin bed is made up of a tiny bead-like material composed of styrene and divinylbenzene. (The actual resin beads are less than 1/32 of an inch in diameter.) The resin beads attract and hold positively charged ions (Na), but will give them up (exchange them) whenever the bead encounters another positively charged ion (Ca and Mg), for which it has greater affinity. Think of it as “The Dating Game.”
- brine tank: This holds the water and dissolved salt solution (brine) that is necessary to perform the ion exchange.
- control valve: It determines the direction of the flow of water through the resin tank or from one location to another during the various softening cycles.

There comes a point when the resin can no longer soften the water. It becomes exhausted. If no new chemical reaction (recharging) is begun, the Ca and Mg ions flow through untouched and the water is no longer softened.
The recharging process
In recharging (regenerating) the water softener, what you’re doing is reversing the ion exchange mention. The Ca and Mg ions are driven off of the resin beads and replaced with Na ions.
Accomplishing this regeneration requires that the resin beads be washed with a strong salt water solution (referred to as “brine”). The excessive concentration of salt forces the Ca and Mg ions to be released and they’re discharged as waste during the backwashing cycle.
The beads are then ready to once again remove Ca and Mg from the water.
Sounds like a lot of work?
In the not too distant past, recharging the softener was a chore. Today, however, the softeners are fully automatic and extremely reliable. The regeneration process comes on automatically, with your only task the occasional filling of the salt storage tank.
Do you need a water softener?
First, you should have your water supply’s hardness level tested. If it’s tested by someone who sells softeners, you can bet you’ll need to buy one. If it’s tested by an independent testing laboratory, or by a firm recommended by your county or municipal health department, maybe not. (You can perform a preliminary hardness test, see T-I-Y section below).
Most manufacturers of water softening equipment will test your water free of charge. (Remember, though, they’re in the business to sell, so be ready to endure the sales pitch.) The salesperson will come to your house, draw a sample of tap water and usually be able to determine the water hardness right in your home.
Another source is through your local health department. They should be able to recommend a testing lab that will provide you with accurate test results for a nominal fee. (A water hardness test usually runs about $20-30). One nice thing about these folks is they usually aren’t trying to sell you a water softener.
What the test tells you
Water hardness is expressed in “grains per gallon” (abbreviated as GPG). The term is a measurement of the number of grains of a given substance in one US gallon of water. Water supplies in this country typically run from 3 to 50 GPG.
- 0 to 1 GPG: soft water
- 1 to 3.5 GPG: slightly hard water
- 3.5 to 7 GPG: moderately hard water
- 7+ GPG: hard or very hard water
The water analysis you have performed will tell you the GPG figure and thus whether a softener is called for.
What size softener
After you’ve learned your water’s GPG, you need to determine your daily household water use before you can choose the right softener. To do this, multiply 75 (average daily per person gallons of water used) by the number of people in your household.
For example, a 4-person household would average 300 gals. per day. Multiplying the 300 gallons x GPG hardness of your Water (20 GPG is common in many areas), gives you a figure of 6,000 GPG total hardness per day.
Water softener capacities are given in terms of the number of grains of hardness they will remove between successive regenerations. The typical household softener’s capacity is between 20,000-30,000 grains. It’s recommended that a softener have enough capacity to last at least three days between regenerations.
Using the example of 6,000 GPG, a 3-day capacity means 18,000 grains. Thus, a softener with a 20,000 resin bed capacity would meet your needs.
Added sodium concerns
The harder the water supply being softened, the more sodium that’s added to the water. If you’re concerned about the added sodium in your body, or if you’re already on a low sodium diet, check with your doctor before installing a water softener.
You can prevent adding sodium to your water intake by having soft water in the hot water line only. Then you’re able to drink and cook with unsoftened, cold water.
T-I-Y: Test It Yourself
There’s a test for water hardness that you can do without fancy equipment. It’s not the most sophisticated test, and you won’t get specific numbers, but you’ll get an idea about the hardness of your water supply.
The test requires three items: a glass jar, with lid; some pure detergent or bar soap (Ivory soap or detergent works well); and unsoftened, cold tap water.
Fill the jar about half-full with cold, unsoftened tap water. Next, sprinkle just a few flakes of detergent, or scrape a few shavings of bar soap into the water. Place the lid securely on the jar and shake the water and detergent/soap until a lather or foam appears on top of the water.
If there’s little or no lather, the water would be considered hard. Now, dip your finger into the water. Hard water will leave soap deposits, or soap curd, on your finger and the inside of the jar. If this is your situation, you’ll probably want your water hardness tested more scientifically.
Magnetize your water for softness? Don’t bet on it
Water conditioning gadgets and gimmicks appear throughout the country on a regular basis. They bring to mind the old “Cure All Elixers.” Use them, and your problems are solved.
Baloney!
The water magnet is one of these oft-promoted wonder cures. It’s attached to the hot or cold water line and claims to magnetically charge the water and lock the hardness minerals in each molecule. For best results, of course, the manufacturer recommends you buy a magnet for both hot and cold lines. Besides, they sell for $400 each!
One advertising illustration actually shows confused water molecules running helter skelter in the water line. But, after they pass through the section of pipe with the magnetizer, they come out in neat little rows, like toy soldiers.
The independent tests on these products show one thing: They don’t work.
There have been numerous tests conducted to check the validity of these claims. The South Dakota School of Mines and Technology found no change in the physical and/or chemical properties of water treated with permanent magnetic devices.
Just remember, if it sounds too good to be true, it probably is.
